Uncovering the Mysterious Structure of Atoms: A Guide for CBSE Class 9 Science Students - Future Classes
Atoms are the building blocks of all matter and are made up of three subatomic particles - protons, neutrons, and electrons. According to CBSE Class 9 Science notes, protons are positively charged, neutrons are neutral, and electrons have a negative charge. Protons and neutrons are located in the nucleus, while electrons are located in shells surrounding the nucleus. The number of protons, neutrons, and electrons in an atom determines the identity of that atom.
Our website offers a free PDF download for CBSE Class 9 Science students to learn about the structure of the atom. The PDF includes detailed diagrams and explanations to help students gain a better understanding of this complex topic. It can be used as a reference guide to review the material, or as a supplement to the classroom curriculum. We hope this resource will help students excel in their science classes.
CHAPTER – 4 STRUCTURE OF ATOM
Dalton assumed that atom is indivisible, i.e., it has no constituent particles. But, a series of experimental evidences revealed that an atom is not the smallest particle.
Some other particles smaller than the atom are also present which are called sub-atomic particles, i.e., electrons, protons and neutrons. The atoms of different elements differ in the number of electrons, protons and neutrons.
Charged Particles in Matter
The particles that carry an electric charge are called charged particles. Generally, on rubbing two objects together, they become electrically charged. It means that some charged particles are present within the atom or atom is made up of some charged particles. Two such particles are electrons and protons.
Discovery of Electrons – Cathode Rays (By J. J. Thomson)
Thomson explained presence of electrons by cathode ray’s experiment.
Facts about Electrons

Discovery of Protons–Anode Rays/Canal Rays (By E. Goldstein)
E. Goldstein by his famous anode rays/canal rays’ experiment was able to detect presence of positively charged particles called protons in the atom.
Facts about Protons

Discovery of Neutrons (By J. Chadwick)
-
J. Chadwick bombarded lighter elements (like lithium, boron etc.) with α-particles and observed emission of new particles having zero charge but having mass equal to that of proton.
-
These particles were called ‘Neutron’ i.e., neutral particle of the atom.
Thomson's Model of an Atom
JJ Thomson was the first scientist to propose a model for the structure of an atom.

The postulates of his model are
-
The mass of an atom is assumed to be uniformly distributed throughout the atom.
-
An atom is considered to be a sphere of uniformly distributed positive charge in which electrons are embedded.
-
The negative and positive charge balance each other therefore, atom as a whole is neutral.
Rutherford’s Model of an atom
-
In his famous ‘α-ray Scattering Experiment’, Rutherford bombarded α-ray (Helium nucleus 2He4) upon thin gold foil.
-
Rutherford made following observations from this experiment:
-
Most of α-particles passed through gold foil undeflected.
-
Some of the α-particles deflected by foil by small angles.
-
One out of every 12000 particles appeared to rebound.

-
On the basis of his experiment, Rutherford proposed model of atom having following features:
-
There is positively placed nucleus in an atom. Nearly all the mass resides in nucleus (Proton + Neutron).
-
Electrons revolves round the nucleus in circular paths.
-
Size of nucleus is very small compared to the size of atom.
-
Drawbacks of Rutherford’s Model
According to Rutherford, electrons revolve round the nucleus in circular paths, but electrons being charged particles will lose their energy and finally will fall into the nucleus. This will make atom highly unstable.
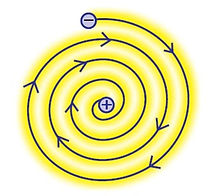
Bohr’s Model of an atom
Neil Bohr proposed modified model of structure of atom. He made following assumptions:
-
Only certain special orbits known as discrete orbits of electrons are allowed inside the atom.
-
While revolving in discrete orbits, the electrons do not radiate energy.
-
These orbits or shells are represented by the letters K, L, M, N, or the numbers, n=1,2,3,4, …

Distribution of Electrons in Various Shells
The distribution of electrons in various shells is done in accordance to ‘Bohr-Bury Scheme’.
The distribution of electrons in various shells is done in accordance to ‘Bohr-Bury Scheme’.
Bohr-Bury Scheme


Valency
-
It is the ability of an atom to gain or lose electron in order to achieve the noble gas configuration.
-
It refers to ability of an element to combine with another element.
Atomic Number
The total number of protons lying in the nucleus of any atom is called the atomic number.

Mass Number
It is the sum of total number of protons and no. of neutrons lying in the nucleus of an atom.

Isotopes
Isotopes are atoms of same elements having same atomic number and different mass numbers.

Uses of isotopes
-
Uranium isotope is used as fuel in nuclear reactor.
-
Isotope of cobalt is useful in treatment of cancer.
-
An isotope of iodine is used in the treatment of goiter.
Isobars
Isobars are the atoms of those elements which have the same mass number but different atomic numbers are called isobars.

